Infection prophylaxis after hematopoietic stem-cell transplant (HSCT) is a standard of care that requires multiple anti-infective agents that target a variety of different pathogens. One particular pathogen of concern is cytomegalovirus (CMV), which is a common viral pathogen that infects the majority of people in the United States but typically does not present with any signs or symptoms of infection in immunocompetent hosts.1 The people who are at risk for clinical CMV infection include infants and patients who are immunocompromised, such as HSCT recipients.1,2 In immunocompromised patients who do not receive prophylaxis, CMV infection can occur in up to 80% of CMV-seropositive patients.2
The consequences of CMV reactivation can lead to significant morbidity and mortality.2 In the setting of allogeneic HSCT, CMV prophylaxis with agents such as ganciclovir and valganciclovir demonstrated efficacy but is not feasible because of significant myelosuppression.3,4
Because of the concern for myelosuppression, clinicians adopted the practice of polymerase chain reaction (PCR) surveillance of CMV as a means of reducing clinical CMV infections.5 PCR surveillance entailed the collection of weekly CMV PCR titers and systemic antiviral treatment was initiated once the CMV PCR titer reached a predefined threshold.5
In November 2017, the US Food and Drug Administration (FDA) approved letermovir for the prophylaxis of CMV infection and disease in CMV-seropositive adults who had undergone an allogeneic HSCT.6 Letermovir is an antiviral agent that inhibits CMV DNA replication by inhibiting the CMV DNA terminase complex, which prevents the packaging of the viral genome into plasmids.6
This novel approach to CMV infection prevention demonstrated efficacy and tolerable safety in clinical trials.7-9 In a phase 3, randomized, multicenter, double-blind, placebo-controlled clinical trial, Marty and colleagues assessed the ability of letermovir to decrease the rate of CMV infection compared with placebo.7 This study enrolled 565 patients, of whom 495 were included in the primary efficacy end point. Study eligibility criteria were age ≥18 years, undergoing allogeneic transplant, and being CMV-seropositive. The primary end point was the proportion of patients with clinically significant CMV infection (defined as the need for preemptive therapy or having CMV disease) at week 24 post-transplant after receiving letermovir or placebo through day 100 post-transplant.7
The results showed that the proportion of patients with clinically significant CMV infection was substantially higher in the placebo group than in the letermovir group (60.6% vs 37.5%, respectively; P <.001).7 Survival was assessed as an exploratory end point and demonstrated a trend toward lower all-cause mortality at week 24 and week 48 post-transplant in the letermovir group versus the placebo group. The safety secondary end points showed no significant difference in the rates of adverse events, except for vomiting and edema, which were higher in the letermovir than the placebo group.7
Since the FDA approval of letermovir, multiple institutions have conducted single-center studies that demonstrated a reduction in CMV viremia with letermovir.8,9 Lin and colleagues conducted a retrospective, single-center review to assess the role of letermovir as primary and secondary prophylaxes in CMV-seropositive HSCT recipients.8 The investigators found a CMV reactivation rate of 5.1% in 39 patients who received primary prophylaxis with letermovir, and no recurrent reactivation in 14 patients who received secondary letermovir prophylaxis.8
Sharma and colleagues compared letermovir prophylaxis with valacyclovir or with valacyclovir followed by acyclovir in CMV-seropositive cord blood transplant recipients.9 In the letermovir group (N = 32), no cases of CMV reactivation occurred compared with a 10% reactivation rate in the valacyclovir group (N = 60) and 22% in the valacyclovir-followed-by-acyclovir group (N = 41). The investigators concluded that letermovir is safe and effective for the primary prophylaxis of CMV reactivation in patients who underwent a cord blood transplant compared with patients who received alternative prophylaxis agents.9
Letermovir was added to the Karmanos Cancer Center’s Detroit, MI, formulary in January 2018 to be used in high-risk allogeneic HSCT recipients starting day 10 after the transplant through day 100 after transplantation. High-risk patients were defined as CMV-seropositive HSCT recipients or CMV-seronegative HSCT recipients with CMV-seropositive donors. The major difference between our patients who were eligible for letermovir prophylaxis and the clinical trial conducted by Marty and colleagues relates to the inclusion of patients who were CMV-seronegative and received a transplant from a CMV-seropositive donor.7
Although patients who are CMV-seronegative and receive stem cells from a CMV-seropositive donor have a lower risk for CMV infection compared with patients who are CMV-seropositive HSCT recipients, according to Ljungman and colleagues there is still an approximate 30% risk for CMV infection in the CMV-seronegative recipients.2 This risk may potentially be further exacerbated by the type of T-cell depletion received during HSCT induction therapy.2
The aim of our study was to assess the real-world application of letermovir for CMV prophylaxis in post-HSCT recipient patients who are CMV-seropositive or CMV-seronegative with a CMV-seropositive donor.
Methods
In this retrospective chart review study we collected data to assess the efficacy of letermovir for CMV prophylaxis in allogeneic HSCT recipients from the electronic medical record (EMR) at a single cancer center, Karmanos Cancer Center. This study was approved by the local Institutional Review Board with a waiver for written informed consent.
The study patients were identified based on letermovir administration data, as well as the utilization of the transplant medication order set. The study inclusion criteria included patients who were aged ≥18 years at the time of their allogeneic HSCT and were either CMV-seropositive or CMV-seronegative with a CMV-seropositive stem-cells donor. The exclusion criteria included severe liver impairment, defined as Child-Pugh class C or creatinine clearance <10 mL/min, as calculated by the Cockcroft-Gault formula. Patients who received an antiviral agent with anti-CMV activity within 90 days before their transplant were also excluded.
The patients were divided into 2 groups. The historical control group included patients who received an allogeneic HSCT between March 1, 2017, and February 28, 2018, namely, before the implementation of the letermovir prophylaxis protocol, and the letermovir group included patients who received letermovir prophylaxis between March 1, 2018, and February 28, 2019, according to the newly instituted standard of care in our institution.
The primary outcome was the relative rate of CMV infection at week 24 after undergoing an HSCT in the historical control group and in the letermovir group. CMV infection was defined as the initiation of an anti-CMV antiviral agent, such as foscarnet, ganciclovir, or valganciclovir, per the Infection Disease Service recommendation or per the standard of care when the CMV PCR test result was >1000 IU/mL.
The secondary outcomes included the characteristics of CMV infection, the rate of clinical CMV infection, the length of hospitalization during the initial transplant, in-hospital mortality, and all-cause mortality at week 24 in the 2 groups. CMV disease was defined as the presence of the appropriate clinical signs and symptoms and/or radiographic findings of CMV or biopsy-confirmed CMV infection plus the detection of CMV by >1000 IU/mL per PCR testing. At our institution, CMV PCR testing is monitored at least weekly.
An additional secondary outcome included the assessment of CMV infection rates in patients with additional high-risk features, such as human leukocyte antigen (HLA) mismatching and significant graft-versus-host disease (GVHD). Significant GVHD was defined as grade ≥2 GVHD requiring the initiation of systemic steroids at a dose equivalent to ≥1 mg/kg daily of prednisone.
The data collected from each patient’s EMR included baseline characteristics; transplant details, such as the conditioning regimen; GVHD prophylaxis; CMV status; donor and matching status; the transplant indication; long-term immunosuppressive therapy; the date of transplant; and the baseline CMV information.
Statistical Analysis
The categorical variables were analyzed using Fisher’s exact test, and the continuous variables between the 2 groups were analyzed using Wilcoxon rank-sum testing. The univariable and multivariable logistic regression analyses were performed among 6 preselected covariates, including donor type; GVHD; stem-cell source; HLA matching; baseline CMV status; and CMV infection. A P value of <.05 was considered statistically significant.
Results
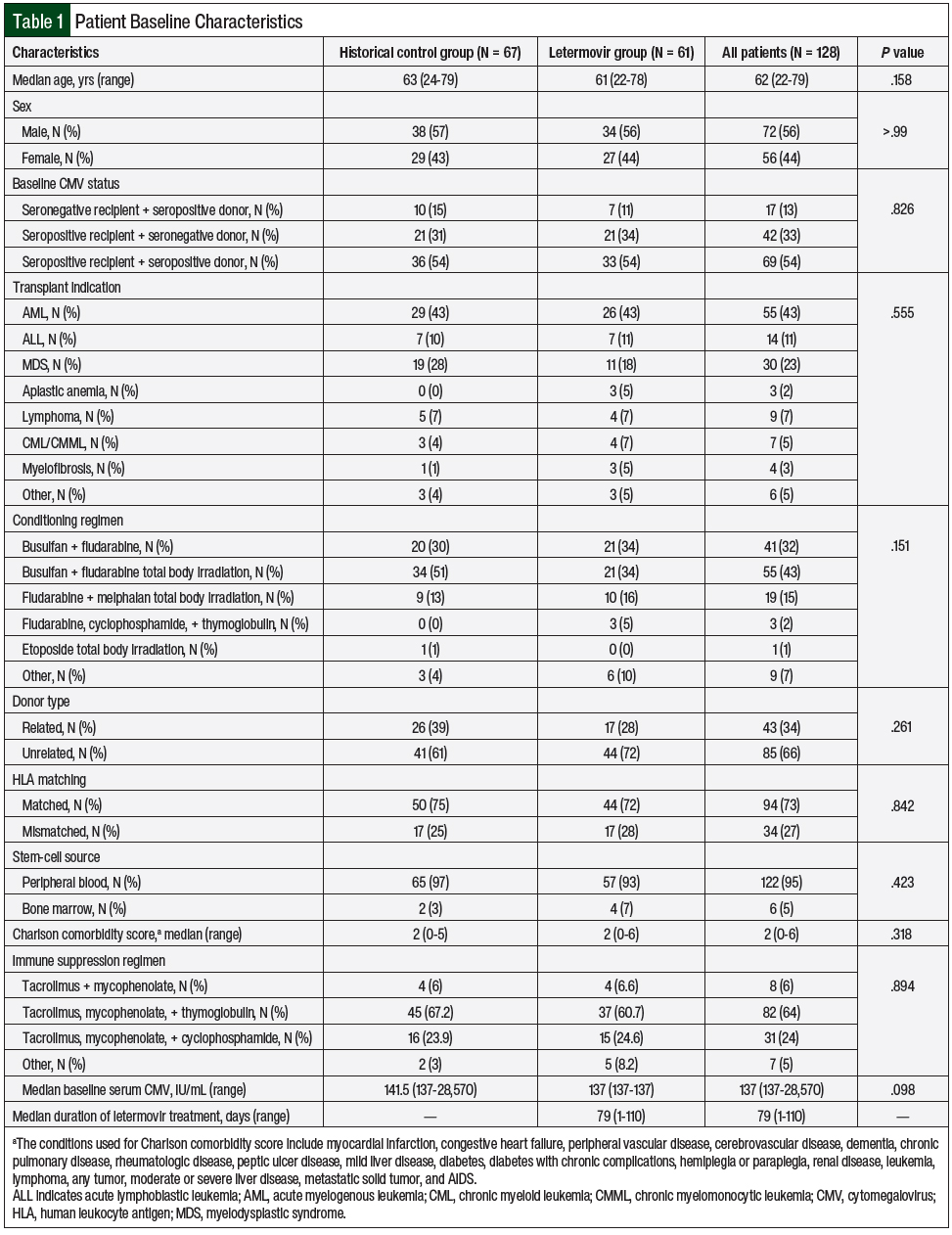
A total of 183 patients received allogeneic HSCT between March 1, 2017, and February 28, 2019, at our institution. In all, 128 patients were identified as eligible for study inclusion—67 patients in the historical control group and 61 patients in the letermovir group (Table 1). Of the 55 patients excluded from the study, the most common exclusion reason was the absence of CMV seropositivity. The baseline characteristics were similar between the 2 groups, with the most common indication for HSCT being acute myelogenous leukemia, and the median age of both groups was 62 years (Table 1).
A total of 55 (43%) patients received a reduced-intensity conditioning regimen of busulfan, fludarabine, and total body irradiation, and 94 (73%) patients received an HLA-matched donor, and matched unrelated donor HSCT transplants were the most common. A further breakdown noted primarily matched unrelated transplants, with 37 patients in the historical group and 38 patients in the letermovir group.
The letermovir group had 6 transplants from a matched related donor and 6 transplants from a mismatch related donor compared with 13 patients with a matched related donor and 4 patients with a mismatch unrelated donor in the control group. All the patients in the letermovir group received either intravenous or oral letermovir 480 mg daily, except for 1 patient who received a 240-mg once-daily dose, because of an interaction with cyclosporine.
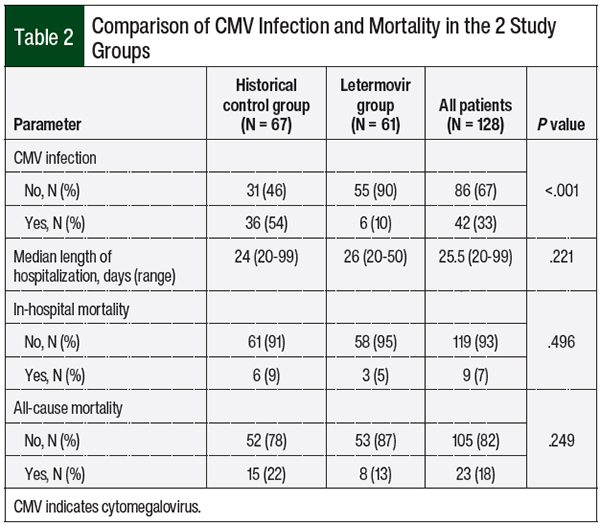
The rate of CMV infection was significantly higher in the control group than in the letermovir group (54% vs 10%, respectively; P <.001; Table 2). Among the CMV-seronegative patients with a CMV-seropositive donor, the rates of CMV infection in the control group and the letermovir group were 30% and 0%, respectively (P = .104). The median duration of letermovir prophylaxis in our study was 79 days. No differences were found between the groups in hospital length of stay, in-hospital mortality, or all-cause mortality at week 24.
Of the patients who had CMV infection, 4 patients in the control group had CMV disease (gastrointestinal manifestation in all 4 cases) compared with no documented CMV disease in the letermovir treatment group (P = .046; Table 3). The time to CMV infection in the letermovir group was significantly longer than in the control group (49.5 days vs 33 days, respectively; P = .039), and a trend toward a shorter duration of CMV treatment was seen in the letermovir group than in the historical controls (18 days vs 27.5 days, respectively; P = .069).
The time to undetectable CMV by PCR was significantly shorter in the letermovir group than the historical control group (16 days vs 29 days, respectively; P = .016). The rate of GVHD (grade ≥2) was not significantly different between the groups (18% in the control group vs 30% in the letermovir group; P = .148).
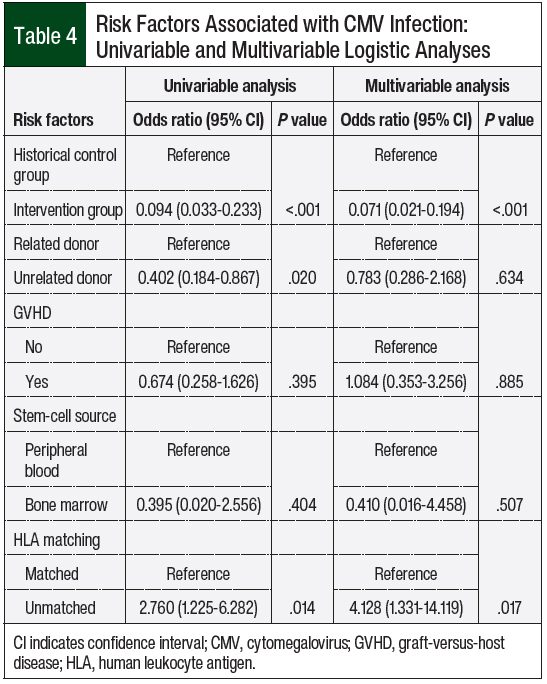
A univariate analysis of risk factors (Table 4) demonstrated that unrelated donors and HLA mismatching, regardless of donor relation, were associated with an increased risk for CMV infection (P = .02). The multivariate analysis of risk factors (Table 4) denoted that HLA mismatch was an independent risk factor for CMV infection (P = .027). Baseline CMV status, significant GVHD, and stem-cell source were not associated with an increased risk for CMV infection.
Discussion
CMV reactivation can result in significant morbidity and mortality in patients undergoing allogeneic HSCT. Based on the phase 3 clinical trial by Marty and colleagues, letermovir offers an effective prophylactic strategy, with minimal adverse events.7
Recently, a retrospective study of patients in Japan reported that letermovir prophylaxis effectively prevented and delayed CMV infection and shortened the duration of anti-CMV treatment post–allogeneic HSCT compared with the nonprophylaxis group.10 Similar to the previously mentioned clinical trials,7,8 our results demonstrated that CMV infection was significantly reduced in patients undergoing HSCT who received prophylaxis with letermovir, although the median duration of therapy was shorter than in either trial. In addition, several single-center studies have demonstrated similar results with letermovir treatment, showing a reduction in the incidence of CMV infection.11-14
The magnitude of the reduction in the CMV infection rate was much larger in our study (44%) than in the pivotal clinical trial by Marty and colleagues (approximately 30%).7 Several single-center retrospective reviews also noted a magnitude of differences in the incidence of CMV infection of more than 30% between their cohorts who did and did not receive letermovir.11,12,14
One aspect that may contribute to this difference is that our institutional GVHD prophylaxis regimens include thymoglobulin, which is a risk factor for CMV reactivation, because of the severe lymphodepletion effect. Thymoglobulin induction was used in 64% of the patients in our study compared with 37.5% of patients in the intervention arm of the study by Marty and colleagues.7
Our study included patients who were CMV-seronegative and received a transplant from a CMV-seropositive donor, which differs from the FDA-approved indication listed in letermovir’s prescribing information.6 In terms of CMV risk factors, the highest risk for CMV infection occurs when the recipient is CMV-seropositive and the donor is CMV-seronegative, because of the lack of donor-transferred CMV-specific antibodies. The lowest risk is when the recipient and the donor are CMV-seronegative; therefore, patients who are CMV-seronegative and receive stem cells from a CMV-seropositive donor or who are CMV-seropositive and receive stem cells from a CMV-seropositive donor fall into the intermediate risk for CMV reactivation.
Our results demonstrated a numerical benefit for the use of letermovir in patients who were CMV-seronegative and received a transplant from a CMV-seropositive donor, although our study was not powered to detect a significant difference in this population. The use of letermovir prophylaxis also significantly delayed the onset of CMV infection and shortened the recovery of CMV to an undetectable level by PCR in our patients. Although it was not statistically significant, the duration of anti-CMV treatment differed by 9.5 days between our 2 groups, which was clinically significant, because the patients were less exposed to medications associated with common adverse effects such as myelosuppression and nephrotoxicity.
Furthermore, our results demonstrated a reduction in the rate of CMV disease in the patients who received letermovir prophylaxis. A trend was seen toward lower all-cause mortality at week 24 among the letermovir group, but this trend did not reach statistical significance. The relatively short follow-up period and the small sample size of this study may contribute to this finding.
The multivariable analysis demonstrated that mismatched HLA was an independent risk factor for CMV infection, but that GVHD (grade ≥2) was not a risk factor, which was reported in previous clinical trials.7,10 Future studies with a larger sample size are needed to determine the full impact of such risk factors.
Limitations
This study was limited by its small sample size, a short follow-up period, and the nature of its retrospective design.
Furthermore, the duration of CMV prophylaxis with letermovir in our institution is from day 10 post-HSCT through day 100 post-HSCT (ie, 91 days total). The median duration of letermovir prophylaxis in our study was 79 days, indicating that there may be some barriers to completing the full course of prophylaxis with this agent.
Conclusion
The addition of letermovir for CMV prophylaxis in patients who had undergone allogeneic HSCT and were CMV-seropositive or CMV-seronegative but received stem cells from a CMV-seropositive donor was effective at preventing and delaying CMV infection. Our results support the expansion of the use of letermovir for CMV prophylaxis beyond the FDA-approved population which only includes CMV-seropositive recipients, not CMV-seronegative patients with a CMV-seropositive donor. Treatment with letermovir may also lead to a decrease in CMV disease and to a shorter duration of anti-CMV treatment.
Further research is needed to determine if certain high-risk patients undergoing HSCT would receive more benefit from letermovir prophylaxis. A future larger-scale study is also needed to assess letermovir compliance rates, in addition to the affordability of letermovir, and how that may affect outcomes, as well as the impact of letermovir compliance and duration of therapy on CMV infection and CMV disease, in patients at high risk or not for CMV reactivation after allogeneic HSCT.
Acknowledgments
We would like to acknowledge Seongho Kim, PhD, Biostatistics Core, Karmanos Cancer Institute, Department of Oncology, School of Medicine, Wayne State University, for providing statistical analysis support.
Author Disclosure Statement
Dr Archambeau, Dr Leece, Dr Patel, and Dr Liu have no conflicts of interest to report.
References
- Centers for Disease Control and Prevention. About cytomegalovirus (CMV). August 18, 2020. www.cdc.gov/cmv/overview.html. Accessed March 14, 2022.
- Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematol Oncol Clin North Am. 2011;25:151-169.
- Goodrich JM, Mori M, Gleaves CA, et al. Early treatment with ganciclovir to prevent cytomegalovirus disease after allogeneic bone marrow transplantation. N Engl J Med. 1991;325:1601-1607.
- Busca A, de Fabritiis P, Ghisetti V, et al. Oral valganciclovir as preemptive therapy for cytomegalovirus infection post allogeneic stem cell transplantation. Transpl Infect Dis. 2007;9:102-107.
- Green ML, Leisenring W, Stachel D, et al. Efficacy of a viral load-based, risk-adapted, preemptive treatment strategy for prevention of cytomegalovirus disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:1687-1699.
- Prevymis (letermovir) tablets, for oral use/injection, for intravenous use [prescribing information]. Merck Sharp & Dohme Corp; February 2021. www.merck.com/product/usa/pi_circulars/p/prevymis/prevymis_pi.pdf. Accessed March 14, 2022.
- Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017;377:2433-2444.
- Lin A, Maloy M, Su Y, et al. Letermovir for primary and secondary cytomegalovirus prevention in allogeneic hematopoietic cell transplant recipients: real-world experience. Transpl Infect Dis. 2019;21:e13187. doi: 10.1111/tid.13187.
- Sharma P, Gakhar N, MacDonald J, et al. Letermovir prophylaxis through day 100 post transplant is safe and effective compared with alternative CMV prophylaxis strategies following adult cord blood and haploidentical cord blood transplantation. Bone Marrow Transplant. 2020;55:780-786.
- Mori Y, Jinnouchi F, Takenaka K, et al. Efficacy of prophylactic letermovir for cytomegalovirus reactivation in hematopoietic cell transplantation: a multicenter real-world data. Bone Marrow Transplant. 2021;56:853-862.
- Johnsrud JJ, Nguyen IT, Domingo W, et al. Letermovir prophylaxis decreases burden of cytomegalovirus (CMV) in patients at high risk for CMV disease following hematopoietic cell transplant. Biol Blood Marrow Transplant. 2020;26:1963-1970.
- Royston L, Royston E, Masouridi-Levrat S, et al. Letermovir primary prophylaxis in high-risk hematopoietic cell transplant recipients: a matched cohort study. Vaccines (Basel). 2021;9:372. doi: 10.3390/vaccines9040372.
- Derigs P, Radujkovic A, Schubert ML, et al. Letermovir prophylaxis is effective in preventing cytomegalovirus reactivation after allogeneic hematopoietic cell transplantation: single-center real-world data. Ann Hematol. 2021;100:2087-2093.
- Anderson A, Raja M, Vazquez N, et al. Clinical “real-world” experience with letermovir for prevention of cytomegalovirus infection in allogeneic hematopoietic cell transplant recipients. Clin Transplant. 2020;34:e13866. doi: 10.1111/ctr.13866.