Telehealth has been defined as “the use of electronic information and telecommunications technologies to support or promote long-distance clinical health care, patient and professional health-related education, public health, and health administration.”1 The benefits of telehealth include increased convenience and access to care, while reducing the constraints of in-person visits, especially for high-risk patients who require frequent monitoring.2,3
Limited evidence suggests that clinical pharmacy telemedicine has an overall positive impact on chronic disease management.2-5 Yet, there is a lack of evidence regarding the impact of telehealth on healthcare utilization, and whether telehealth services should function as a substitution for or as a complement to in-person visits.6
In March 2020, the Coronavirus Preparedness and Response Supplemental Appropriations Act was enacted, which loosened restrictions on telehealth services.7 In addition, the Centers for Medicare & Medicaid Services (CMS) expanded its telehealth waiver to allow for the management of conditions unrelated to COVID-19 to be conducted via telehealth.8 This waiver also states that telehealth services can be delivered by nonphysician qualified health professionals, listing pharmacists as auxiliary personnel.8
Despite these federal regulations, challenges remain for the compensation of pharmacy services because pharmacists are not recognized as providers but are considered “auxiliary personnel” by CMS.9-11 Under the CMS waiver, auxiliary personnel can deliver and bill for certain services, such as evaluation and management, under the direct supervision of a physician as part of a bundled payment.9 The physician can then make payments to the auxiliary personnel (eg, the pharmacist) based on the terms of any financial agreements.12
Pharmacists are recognized as highly qualified, yet underutilized, healthcare professionals.2 Pharmacist-led oral chemotherapy management reduces the rates of medication-related adverse events, drug interactions, and medication errors, as well as improves adherence and patient safety, and reduces healthcare costs.13-15 Most of the evidence describing the outcomes of pharmacists in the provision of telehealth services involves the management of chronic diseases, such as high blood pressure and diabetes.4,16-18 These studies have demonstrated better achievement of blood pressure and hemoglobin A1c goals compared with usual care, as well as enhanced pharmacist clinical services.4,16-18
Follow-up phone calls have been a part of pharmacist practice for years as part of Medicare Part D medication therapy management (MTM), comprehensive medication management, and education services17,19-23; however, there is a paucity of studies regarding the outcomes of pharmacist involvement in the delivery of expanded telehealth services as a result of the COVID-19 pandemic, particularly in the oncology setting.24 Patients with cancer have a higher risk for complications from COVID-19 compared with patients without cancer,25,26 and delaying cancer treatments is often not feasible; therefore, telehealth services are necessary to ensure that patients have safe access to quality care without delay.
At the onset of the COVID-19 pandemic, in-person medical visits were severely restricted at our institution, which created an urgent and sustained need for telehealth services for the provision of usual pharmacist care, such as chemotherapy education and adverse event monitoring. At the University of Kansas Cancer Center, pharmacists are an integral part of the cancer care team in multiple cancer subspecialties. Pharmacist involvement in the gynecologic oncology clinic has been long-standing and serves as a model for many of our other clinical practices. With the swift rollout of telehealth and rapidly evolving workflows, we chose to track outcomes specifically in the gynecologic oncology clinic, although many of these services and outcomes could be applied to other specialty clinic areas as well.
In our ambulatory oncology clinics, pharmacists provide intravenous (IV) and oral cancer therapy education for chemotherapy, immunotherapy, and targeted therapy; perform adverse event checks; serve as drug information resources; and assist in managing cancer therapy–related adverse events. Before the COVID-19 pandemic, most of the pharmacist–patient encounters in the gynecologic oncology clinic occurred during the patient’s regularly scheduled office visit. All patients starting a new cancer therapy were scheduled for an in-person education session with the pharmacist, followed by a cancer therapy consent visit with the advanced practice provider (APP).
Pharmacists joined the APPs and physicians during other in-person visits to provide IV and oral cancer therapy adverse event checks and reassessments. As soon as telehealth services became available at the University of Kansas Cancer Center, APP consent visits, along with many other appointment types, transitioned to telehealth appointments; consequently, the pharmacist team also had to adapt its approaches to patient education and follow-up.
In addition to the need for telehealth services to provide care during the COVID-19 pandemic, provider shortages at our institution exposed an opportunity for pharmacists to play a role in the optimal care of patients receiving IV and oral cancer therapies. Provider shortages are not unique to our institution; the American Society of Clinical Oncology projects that there will be a shortage of more than 2200 oncologists by 2025 in addition to an increased demand for cancer care.27
Pharmacists were identified by the gynecologic oncology providers to assist with adverse event checks of patients receiving targeted therapies, immune checkpoint inhibitors, or oral cancer therapies. Patients were scheduled with a pharmacist in place of a provider before their next planned treatment to determine if it was appropriate to proceed with IV treatment or if they should continue receiving oral cancer therapy. Pharmacists provided many of these services via telehealth technologies, but in-person visits still occurred for some patients during the study period and are not included in our study data.
The rapid incorporation and adoption of telehealth services within our health system created a unique opportunity for pharmacist involvement in expanded telehealth services. The objectives of this study were to demonstrate the feasibility of and characterize the impact of pharmacist-provided telehealth services within an ambulatory oncology clinic, and to assess the utilization of pharmacists with the integration of telehealth at our institution.
Methods
This was a single-center, prospective, observational study conducted at our National Cancer Institute–designated University of Kansas Cancer Center. Patients aged ≥18 years who participated in gynecologic oncology pharmacist telehealth encounters between August 5, 2020, and December 17, 2020, were included in the study. Patients were excluded if they could not be reached for their telehealth visit, if their visit was canceled, or if they declined follow-up with a pharmacist.
Four clinical pharmacists with training in the gynecologic oncology clinic completed all the telehealth appointments included in the study. Pharmacists received training on how to use the videoconferencing application and appropriate telehealth conduct. Telehealth visits were completed via telephone or via videoconferencing.
Our institution uses our electronic medical record (EMR), Epic, for scheduling telehealth visits. Patients were scheduled on the existing gynecologic oncology pharmacist template in advance of their appointment time and were sent a link through MyChart—a secure electronic patient messaging platform integrated into our EMR—to access the telehealth appointment via the Zoom videoconferencing application, if applicable. Consent for telehealth was obtained at the time of patient scheduling, and written consent materials were provided to patients in their appointment reminder that was sent through MyChart.
Oral and IV cancer therapy telehealth education occurred before the patient’s first treatment cycle. Oral cancer therapies included poly (ADP-ribose) polymerase (PARP) inhibitors, tyrosine kinase inhibitors (TKIs), and mammalian target of rapamycin (mTOR) inhibitors. IV cancer therapies included chemotherapy (taxanes, platinum drugs, antimetabolites), targeted therapy (bevacizumab), and immunotherapy (pembrolizumab). Patients were educated on the treatment schedule, potential adverse events, supportive medications, and reasons to contact the provider’s office or to seek immediate medical attention.
Medication reconciliation and review were also conducted. Oral cancer therapy administration instructions, the importance of treatment adherence, and handling precautions were reviewed during education sessions. The anticipated monitoring and follow-up schedules were provided to the patient. The patients were provided written education materials via MyChart messages. Screen sharing was used during videoconferencing sessions to assist in educating patients about the written materials to mimic in-person education sessions.
Telehealth oral cancer therapy adverse event checks occurred within 2 weeks of initiating treatment. Formal oral cancer therapy reassessments occurred every 3 months or on an as-needed basis, per provider request. Pharmacists monitored laboratory values in-between reassessment visits, but they did not complete telehealth visits for laboratory-only monitoring.
Adverse event checks and reassessments included a review of medication adherence, adverse events, and changes to concomitant medications, as well as a review of any pertinent laboratory tests. Reassessments included an added review of clinical response to therapy. Pharmacists facilitated prescription acquisition and functioned as a liaison between the patient and the specialty pharmacy to obtain high-cost medications at an affordable price. Existing education and reassessment progress note templates were used to document IV and oral cancer therapy education, as well as oral cancer therapy adverse event checks.
Telehealth IV and oral cancer therapy adverse event checks in place of an in-office provider visit occurred in patients whose disease had been stable after receiving treatment for more than 6 months and were deemed appropriate to receive follow-up care by a pharmacist, per the physician’s discretion. The medications included bevacizumab, pembrolizumab, PARP inhibitors, TKIs, and mTOR inhibitors, or a combination of IV and oral therapies. Patients could decline a telehealth or in-office pharmacist visit in place of an in-office provider visit, in which case they would be scheduled for a visit with a physician or an APP instead.
These telehealth visits were completed before a patient’s scheduled IV cancer therapy infusion appointment or a subsequent cycle of oral therapy, and were considered a pharmacist visit in place of a provider visit. A standard progress note in a SOAP (subjective, objective, assessment, plan) format was created to complete and document the adverse event checks. The pharmacists assessed the adverse events, completed a review of systems, and reviewed laboratory test results to ensure that patients were eligible to receive IV treatment or to continue with oral therapy.
Recommendations and urgent interventions were discussed with the covering APP or physician. On completion of the telehealth visit, the pharmacist would document a progress note in the EMR and route the note to the physician and/or the APP.
To characterize the impact of the pharmacist-provided telehealth services, we assessed the implementation rate of pharmacist recommendations. The recommendations were classified as the identification of prescribing problems related to oral or IV cancer therapy, the identification of IV or oral cancer therapy adverse events that required an intervention, or the recommendation for additional antiemetic therapy for cancer therapies that are deemed to have moderate or high emetic risk.
We also assessed the identification of significant drug interactions and the rate of adherence to oral cancer therapy. Significant drug interactions were defined as Category D (“consider therapy modification”) or higher and were discussed with the provider. The oral cancer therapy adherence rate was defined as “no missed doses,” “1 missed dose,” or “more than 1 missed dose” during the follow-up period. We characterized the impact of telehealth on healthcare utilization, by determining if the telehealth visit was a service that was being delivered before our telehealth implementation, if it replaced a similar in-person service, and/or if it supplemented an in-person service.
The University of Kansas Health System Institutional Review Board committee approved our study. The study data were collected and stored in the University of Kansas Health System REDCap server.
Results
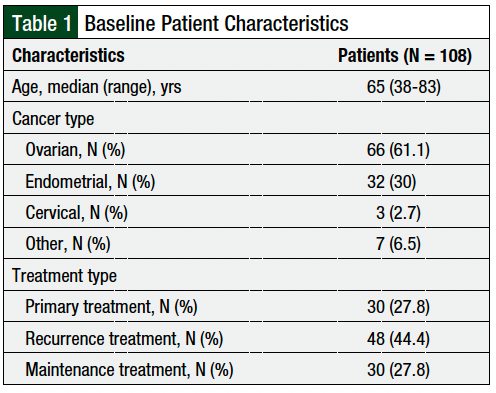
From August 5, 2020, to December 17, 2020, a total of 108 patients from the gynecologic oncology clinic completed telehealth appointments with a clinical pharmacist and were included in our study (Table 1). The patients’ median age was 65 years. The patients predominately had ovarian cancer (61.1%) or endometrial cancer (30%). The patients were receiving therapy for disease recurrence (44.4%), primary treatment (27.8%), or maintenance treatment (27.8%; Table 1).
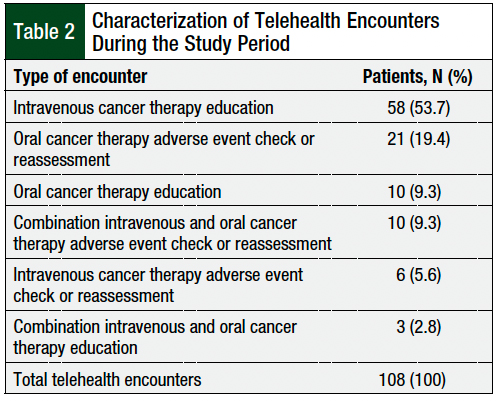
The most common pharmacist telehealth encounters were for IV cancer therapy education (53.7%), followed by oral cancer therapy adverse event checks or reassessments (19.4%), oral cancer therapy education (9.3%), and combination IV and oral cancer therapy adverse event checks or reassessments (9.3%; Table 2). Overall, 68% of the telehealth visits were completed via telephone, and 32% of the telehealth visits were completed via videoconferencing.
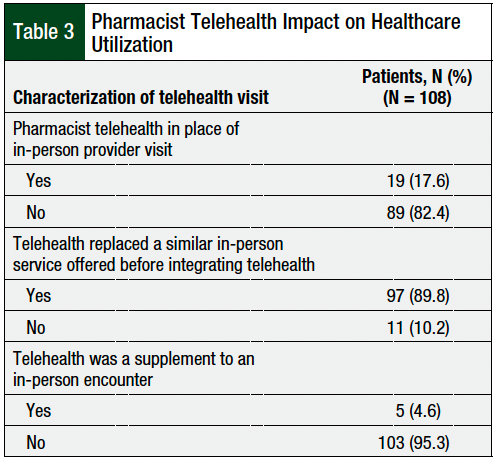
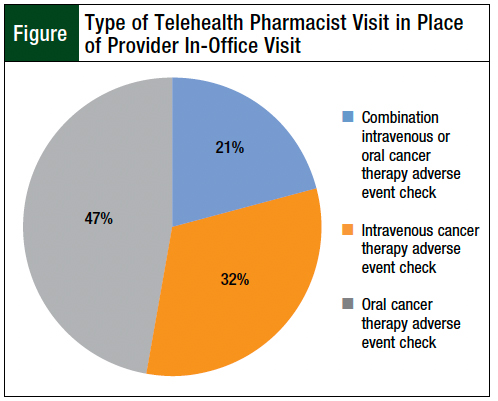
During the study period, 89.8% of the services offered were previously delivered as in-person services before the incorporation of telehealth (Table 3). Pharmacist visits occurred in place of a regular provider visit in 17.6% of telehealth encounters (Table 3). Oral and IV cancer therapy adverse event checks were the services performed in place of a regularly scheduled provider visit (Figure).
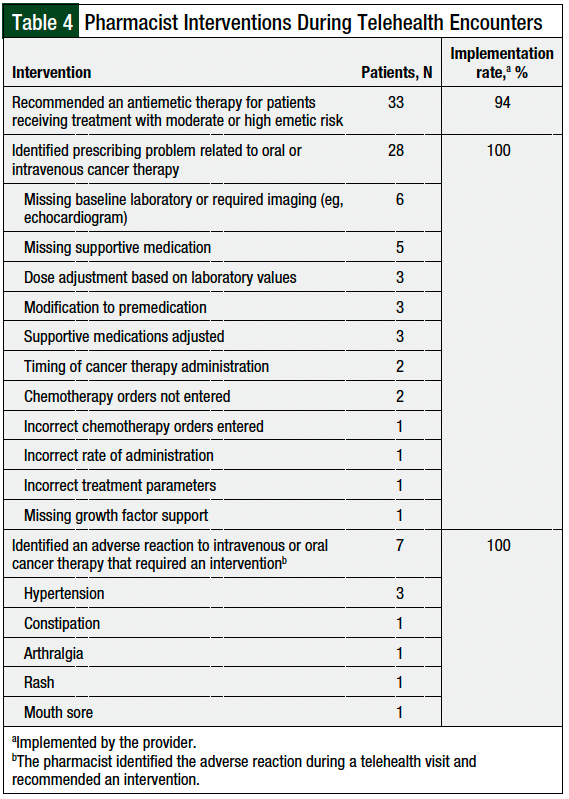
Pharmacists recommended an antiemetic therapy for 33 patients receiving IV or oral cancer therapies that were associated with moderate to high emetic risk, and the recommendation was implemented by providers in 94% of the cases (Table 4). The majority of antiemetic therapies recommended were for the treatment of breakthrough nausea in patients receiving a platinum-based chemotherapy regimen.
Oral or IV cancer therapy–related prescribing problems were identified during 28 (25.9%) encounters. Missing baseline laboratory tests or required baseline imaging, such as an echocardiogram or electrocardiogram (N = 6); missing supportive medications, such as IV diphenhydramine, to prevent infusion reactions (N = 5); and the need for a dose adjustment based on renal, hepatic, or other laboratory values (N = 3), were among the top prescribing problems identified by pharmacists during telehealth encounters (Table 4). Pharmacist recommendations to correct the prescribing problem were accepted by the providers in 100% of the patients (Table 4).
The identification of an adverse reaction to IV or oral cancer therapy requiring an intervention occurred during 7 patient encounters, and in all these cases the providers accepted the pharmacist’s recommendations. Hypertension was the most frequently associated adverse event that required an intervention (Table 4).
Adherence to oral cancer therapy was assessed during 30 of 31 oral or combined IV and oral cancer therapy adverse event checks or reassessments. During the follow-up period, 90% of the patients were adherent to their medication and had ≤1 missed doses.
Discussion
The COVID-19 pandemic led to a rapid shift in the delivery of care for patients with cancer. Swift changes to federal regulations assisted in the prompt adoption of, and increased access to, telehealth services. Limitations to in-person visits and the enforcement of social distancing protocols led to the prioritization of telehealth visits for appropriate patients. Our institution quickly transitioned to integrate Zoom telehealth visits into the Epic system, which allowed patients and healthcare personnel to communicate using Health Insurance Portability and Accountability Act–compliant technology.28,29
As soon as telehealth services were available at our institution, providers were encouraged to transition as many appointments as possible to telehealth. Pharmacists are an integral part of the oncology care team, and all patients must receive pharmacist-led education before initiating new treatments; therefore, it was essential that pharmacists’ visits also transition to telehealth to ensure the continuity of care. Our study specifically tracked pharmacist involvement within the gynecologic oncology clinic, but our results could be applied to other clinical specialties as well.
In our study, pharmacists were rapidly able to implement and provide telehealth services to patients with cancer receiving IV and/or oral cancer therapies. As expected, most encounters (89.8%) replaced in-person services during the study period, demonstrating that pharmacists can provide similar services via telehealth, without compromising patient care. This also highlights that before the integration of telehealth, most patient–pharmacist encounters were in person, and only a small percentage of pharmacist follow-up was being completed via telephone, indicating a significant workflow change with the integration of telehealth. Moreover, the implementation of telehealth allowed for the provision of supplemental pharmacist services, such as increased adverse event monitoring of IV and oral cancer therapies.
The use of telehealth resulted in the expansion of the role of the clinical pharmacist, as well as increased access to care for patients. New services, such as IV and oral cancer therapy adverse event checks in place of an in-office provider visit, were delivered because of the integration of telehealth into our institution. These were conducted at in-person and at telehealth pharmacist visits. Our study only tracked data on telehealth encounters, so the actual increased number of pharmacist services delivered during the study period is higher than what we report. This important new service has increased the use of pharmacists in the clinical setting and has eased the scheduling challenges caused by ongoing provider shortages.
During our study period, pembrolizumab and bevacizumab were the only IV medications for which pharmacists were seeing patients in place of a provider to clear them for treatment. As a result of our involvement, this practice has now expanded to include patients receiving single-agent and combination IV chemotherapies, such as carboplatin, paclitaxel, and gemcitabine, to clear patients to receive their next dose of IV treatment.
Our data indicate that telehealth services can function as a substitution for, and as a complement to, in-person visits. Supplemental visits with the pharmacist were offered and were delivered to patients in addition to their regularly scheduled provider visits. This can be particularly helpful for patients who are receiving highly toxic regimens for cancer or those who require close follow-up and monitoring, such as patients receiving PARP inhibitors or a combination of a TKI and an immunotherapy. Patients with low health literacy who require reinforcement of education and patients taking oral cancer therapies at home were among those who benefited from supplemental pharmacist telehealth visits.
Pharmacists functioned as physician extenders at times of provider shortages during the study, which are still ongoing; approximately 18% of visits in our study were a pharmacist telehealth visit that replaced an in-office provider visit. Pharmacist availability ameliorated the scheduling challenges introduced from the backlog of patients who were rescheduled beginning with the onset of the pandemic. This is especially important, because current projections estimate that by 2033, physician demand will grow faster than the physician supply, leading to a shortage of between 54,100 and 139,000 providers.30 Kansas pharmacy law does not recognize pharmacists with provider status31; therefore, we were unable to bill for our services rendered. Furthermore, Kansas has not adopted legal definitions of or requirements for MTM services.32
These factors underscore the need for the expansion of provider status for pharmacists in all states, to ensure compensation for value-added pharmacist services. As part of the regulations set forth by CMS in response to COVID-19, pharmacists can “provide and bill for telehealth services rendered ‘incident to’ services provided by a Medicare-eligible provider.”10 Pharmacists at our cancer center, however, are not practicing under a collaborative practice agreement, nor have we established any financial agreements to receive payment as part of a bundled service payment. (Bundled service payment represents a potential opportunity to receive payment for pharmacist services.)
Our study shows that pharmacists improved symptom management by optimizing antiemetic regimens before the receipt of cancer therapies. As a result of the high number of antiemetic regimens recommended for breakthrough nausea in our study, our chemotherapy templates will be undergoing revisions to optimize regimens for breakthrough nausea.
The identification of cancer therapy–related prescribing problems in 26% of telehealth encounters demonstrates that pharmacists serve as an important safety check before the receipt of chemotherapy, immunotherapy, or targeted therapies. Identifying prescribing problems in advance of a patient’s scheduled treatment avoids delays in care and can potentially prevent adverse reactions to therapy, such as hypersensitivity reactions, which may occur if appropriate premedication is not ordered.
Three patients in our study did not have chemotherapy orders entered at the time of the telehealth visit or did not have the correct chemotherapy orders entered. The pharmacists were able to identify and resolve these issues before the beginning of the patients’ scheduled treatment.
Several patients also had adverse reactions to their cancer therapy that warranted an intervention by the provider, and were discovered during the pharmacist telehealth visit. Pharmacists recommended hypertension management for 3 patients receiving targeted therapies. The providers implemented all pharmacist recommendations for the management of the identified adverse events. The high implementation rate for pharmacist recommendations in our study demonstrates that physicians trust pharmacists and agree with the interventions they recommend in the delivery of care to patients with cancer.
In our study, pharmacist-provided oral chemotherapy education and follow-up resulted in higher levels of adherence than were previously described in pharmacist-managed oral chemotherapy programs before the pandemic.13,14 Telehealth appointments offer a convenient way for pharmacists to perform more frequent assessments and to identify barriers to medication adherence. Because our patients were in their homes, they had access to their medications, could show the pharmacists the pills if needed, and could provide accurate medication histories.
Our study demonstrates that pharmacist services can also be delivered via telehealth to provide comprehensive pharmacist care to patients with cancer. As a result of telehealth visits, pharmacists served as a safety check to ensure that cancer therapies were prescribed appropriately, including all appropriate baseline monitoring and supportive therapy before the delivery of therapy, and ensured that patients who had adverse reactions to IV and/or oral cancer therapies were monitored and managed appropriately.
The incorporation of telehealth and in-person visits within the same pharmacist template offered patients more choices when scheduling medical appointments, prevented overbooking of the pharmacist, and improved our ability to reach patients, because routine telephone follow-up was now on the pharmacist template. This created a sustainable and feasible practice model to continue the use of pharmacist-provided telehealth in the ambulatory setting.
When scheduling a pharmacist telehealth visit, patients were offered the choice to complete a visit by telephone or by videoconference. At first, many patients were reluctant to schedule videoconference visits, because of its novel use and their unfamiliarity with the technology, and many of our visits were conducted by telephone. As telehealth has become more prevalent and widely used at our institution, a much higher rate of patients are now scheduling videoconferencing follow-up. In the 4.5 months after our study period, pharmacist telehealth appointments in the gynecologic oncology clinic have increased to an average of 28 visits per month—an increase from an average of 24 telehealth visits per month during our study period—and 75% of those visits have used videoconferencing.
Telehealth has added benefits to in-person visits. Patients could invite family members, including distant members, to join the telehealth appointments, at a time when visitors were restricted onsite at the University of Kansas Cancer Center. Telehealth appointments can also be more convenient for patients who live in rural areas, or those who have transportation and mobility barriers. Telehealth offers more flexibility for patients if they do not have to commute to an in-person visit, find childcare, find work coverage, or other time-constraining considerations that arise with an overall longer in-person visit.
Limitations
Our study has some limitations. Because telehealth is a new service at our institution that was instituted during the COVID-19 pandemic, when in-person visits were severely limited, we did not track pharmacist encounters or interventions that were delivered in person during the study period. Hence, we were unable to compare the pharmacist services delivered via telehealth directly with those delivered via in-person appointments.
In addition, before this study, we were not routinely tracking pharmacists’ interventions; consequently, we have limited data available on the impact of in-person pharmacist encounters.
Finally, we did not assess the length of time for each telehealth visit, which would have been an interesting outcome to quantify and compare with the length of time of an in-person visit.
Conclusion
Pharmacists were able to implement rapidly and provide telehealth services to patients with cancer who were receiving IV or oral cancer therapies. Utilization of telehealth expanded the role of the clinical pharmacist and increased access to care for patients. The use of pharmacist-provided telehealth services resulted in improved symptom management and the prevention of prescribing errors. These findings suggest that telehealth services can function as a substitution for, and a complement to, in-person visits. The incorporation of pharmacist-provided telehealth services into an ambulatory clinic is an achievable practice model.
Author Disclosure Statement
Dr Bohnenkamp, Dr Fahey, and Dr Hefler have no conflicts of interest to report. Dr Martin is on the G1 Therapeutics Advisory Board. Dr Dubois is on the Speaker’s Bureau of Pfizer and was on the Eisai Advisory Board.
References
- American Pharmacists Association. Telehealth. 2020. www.pharmacist.com/Practice/Practice-Resources/Telehealth. Accessed December 11, 2020.
- Niznik JD, He H, Kane-Gill SL. Impact of clinical pharmacist services delivered via telemedicine in the outpatient or ambulatory care setting: a systematic review. Res Social Adm Pharm. 2018;14:707-717.
- Badowski ME, Walker S, Bacchus S, et al. Providing comprehensive medication management in telehealth. Pharmacotherapy. 2018;38:e7-e16.
- Taylor AM, Bingham J, Schussel K, et al. Integrating innovative telehealth solutions into an interprofessional team-delivered chronic care management pilot program. J Manag Care Spec Pharm. 2018;24:813-818.
- Omboni S, Tenti M, Coronetti C. Physician–pharmacist collaborative practice and telehealth may transform hypertension management. J Hum Hypertens. 2019;33:177-187.
- Shigekawa E, Fix M, Corbett G, et al. The current state of telehealth evidence: a rapid review. Health Aff (Millwood). 2018;37:1975-1982.
- Coronavirus Preparedness and Response Supplemental Appropriations Act. H.R.6074 (2020). www.congress.gov/bill/116th-congress/house-bill/6074/text. Accessed September 20, 2021.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. March 17, 2020. www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Accessed September 20, 2021.
- Centers for Medicare & Medicaid Services, HHS. Medicare and Medicaid programs, basic health program, and exchanges; additional policy and regulatory revisions in response to the COVID-19 public health emergency and delay of certain reporting requirements for the skilled nursing facility quality reporting program. Interim final rule with comment period. www.govinfo.gov/content/pkg/FR-2020-05-08/pdf/2020-09608.pdf. Accessed September 20, 2021.
- Segal EM, Alwan L, Pitney C, et al. Establishing clinical pharmacist telehealth services during the COVID-19 pandemic. Am J Health Syst Pharm. 2020;77:1403-1408.
- Bonner L. Pharmacists embrace telehealth during COVID-19. Pharmacy Today. 2020;26:26-29.
- Centers for Medicare & Medicaid Services. Physicians and other clinicians: CMS flexibilities to fight COVID-19. March 30, 2020. www.endocrine.org/-/media/endocrine/files/membership/cms--covid-phys-fact-sheet.pdf. Accessed September 20, 2021.
- Wong SF, Bounthavong M, Nguyen C, et al. Implementation and preliminary outcomes of a comprehensive oral chemotherapy management clinic. Am J Health Syst Pharm. 2014;71:960-965.
- Battis B, Clifford L, Huq M, et al. The impacts of a pharmacist-managed outpatient clinic and chemotherapy-directed electronic order sets for monitoring oral chemotherapy. J Oncol Pharm Pract. 2017;23:582-590.
- Muluneh B, Schneider M, Faso A, et al. Improved adherence rates and clinical outcomes of an integrated, closed-loop, pharmacist-led oral chemotherapy management program. J Oncol Pract. 2018;14:e324-e334.
- Murry LT, Parker CP, Finkelstein RJ, et al. Evaluation of a clinical pharmacist team-based telehealth intervention in a rural clinic setting: a pilot study of feasibility, organizational perceptions, and return on investment. Pilot Feasibility Stud. 2020;6:127. doi: 10.1186/s40814-020-00677-z.
- Maxwell LG, McFarland MS, Waymier Baker J, Cassidy RF. Evaluation of the impact of a pharmacist-led telehealth clinic on diabetes-related goals of therapy in a veteran population. Pharmacotherapy. 2016;36:348-356.
- Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310:46-56.
- Johnson M, Jastrzab R, Tate J, et al. Evaluation of an academic-community partnership to implement MTM services in rural communities to improve pharmaceutical care for patients with diabetes and/or hypertension. J Manag Care Spec Pharm. 2018;24:132-141.
- DeZeeuw EA, Coleman AM, Nahata MC. Impact of telephonic comprehensive medication reviews on patient outcomes. Am J Manag Care. 2018;24:e54-e58.
- Gershman J. Telehealth offers myriad unique opportunities for pharmacists. Pharmacy Times. April 2020;88. www.pharmacytimes.com/view/telehealth-offers-myriad-unique-opportunities-for-pharmacists. Accessed August 3, 2021.
- Centers for Medicare & Medicaid Services. Telehealth. www.medicare.gov/coverage/telehealth. Accessed February 7, 2020.
- Ai AL, Carretta H, Beitsch LM, et al. Medication therapy management programs: promises and pitfalls. J Manag Care Spec Pharm. 2014;20:1162-1182.
- Yerram P, Thackray J, Modelevsky LR, et al. Outpatient clinical pharmacy practice in the face of COVID-19 at a cancer center in New York City. J Oncol Pharm Pract. 2021;27:389-394.
- Yemm KE, Arnall JR, Cowgill NA. Necessity of pharmacist-driven nonprescription telehealth consult services in the era of COVID-19. Am J Health Syst Pharm. 2020;77:1188.
- Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335-337.
- Yang W, Williams JH, Hogan PF, et al. Projected supply of and demand for oncologists and radiation oncologists through 2025: an aging, better-insured population will result in shortage. J Oncol Pract. 2014;10:39-45.
- Zoom. HIPAA Compliance Datasheet. https://explore.zoom.us/docs/doc/Zoom-hipaa.pdf. Accessed September 20, 2021.
- Centers for Medicare & Medicaid Services, HHS. Health insurance reform: security standards. Final rule. www.govinfo.gov/content/pkg/FR-2003-02-20/pdf/03-3877.pdf. Accessed September 20, 2021.
- Association of American Medical Colleges. The Complexities of Physician Supply and Demand: Projections from 2018 to 2033. Washington, DC; June 2020. www.aamc.org/media/45976/download. Accessed January 4, 2021.
- Kansas Board of Pharmacy. Laws and Regulations. Updated February 2020. https://pharmacy.ks.gov/docs/librariesprovider10/statues-regulations/full-version-pdf.pdf?sfvrsn=66fca701_20. Accessed September 23, 2021.
- Thomas D, Tran J. Medication therapy management. In: Thomas DJ, Tran J, eds. The Medication Therapy Management Pharmacist Reference Book: A Comprehensive Review of Medication Therapy Management in the United States. National Board of Medication Therapy Management; September 1, 2020. https://nbmtm.org/mtm-reference/medication-therapy-management/. Accessed August 3, 2021.