Autologous hematopoietic-cell transplant (HCT) has been a mainstay in the treatment of patients with relapsed lymphoma for decades.1 Before autologous HCT, adequate mobilization and collection of hematopoietic cells are critical to ensuring successful stem-cell rescue after the administration of high-dose chemotherapy.1 The reinfusion of ≥2 × 106 CD34+ cells/kg reduces the risk for graft nonresponse and improves the likelihood of adequate neutrophil and platelet engraftment, with increased CD34+ cell doses associated with faster neutrophil and platelet engraftments.2-4
Mobilization is the process of stimulating production of CD34+ multipotent stem cells from the bone marrow and subsequently releasing them into the peripheral blood before apheresis; however, sometimes an adequate cell dose is not collected.5 Chemomobilization involves the administration of chemotherapy in addition to granulocyte colony-stimulating factor (G-CSF) and may result in greater CD34+ stem-cell collection yields than with G-CSF alone.5 Furthermore, plerixafor may be given as part of the chemomobilization strategy to improve stem-cell collection yield.2,3,5
The University of North Carolina Medical Center has found that the implementation of an algorithm-based approach to plerixafor utilization in the setting of chemomobilization, driven by CD34+ cell count and collection goals, is a favorable and cost-effective approach; however, institutional mobilization protocols for autologous transplantation may vary.5,6
Although many chemomobilization regimens are available, which agents and doses are the most effective remains unclear.3,4 Etoposide is an effective agent for chemomobilization in lymphoma and was the drug of choice at our institution until a national etoposide shortage in February 2018 prompted our institution, the University of North Carolina Medical Center, to switch to cyclophosphamide as the preferred chemomobilization agent.7
No consensus exists about the optimal cyclophosphamide dose for chemomobilization; doses as low as 1 to 1.5 g/m2 have been used in patients with lymphoma to collect the minimum number of CD34+ cells required to proceed to transplant.5,8,9 Higher doses of cyclophosphamide (3-7 g/m2) have also been used and have been associated with higher CD34+ cell yields and lower nonresponse rates, but with increased adverse events and costs.2,5
Our institution selected a conservative, 1-time dose of 2 g/m2 administered intravenously, with hydration and mesna support, in an attempt to minimize the risk for cyclophosphamide-related adverse drug reactions, such as hemorrhagic cystitis and prolonged neutrophil and platelet recovery, without compromising successful stem-cell collection. Etoposide- and cyclophosphamide-based chemomobilization regimens are associated with increased hospitalizations for neutropenic fever compared with G-CSF–based mobilization alone.3,10
The goal of this noninferiority study was to compare the efficacy and adverse reactions profiles of cyclophosphamide and etoposide to identify a preferred chemomobilization agent for future use at our institution.
Methods
This was a single-center, retrospective analysis of patients with lymphoma who were undergoing cyclophosphamide- or etoposide-based first mobilization before autologous HCT between September 1, 2016, and August 31, 2019. Patients were included if they were aged ≥18 years at the time of chemomobilization, and had received a single dose of either cyclophosphamide 2 g/m2 intravenously or etoposide 300 mg/m2 intravenously, daily, for 2 doses as mobilizing chemotherapy.
All administration of cyclophosphamide and etoposide was done in the outpatient setting. Patients who had cyclophosphamide-based mobilization received 2 doses of intravenous (IV) mesna 400 mg/m2: 1 dose 15 minutes before the start of the cyclophosphamide infusion, and 1 dose 2 hours after the end of the cyclophosphamide infusion. In addition, patients were prescribed 1 dose of oral mesna 800 mg/m2 to take 6 hours after the end of the cyclophosphamide infusion.
Patients who received cyclophosphamide-based mobilization also received 3 IV sodium chloride 0.9% 500-mL boluses: the first 2 given before and after the cyclophosphamide dose, and the third given the next day. Patients received G-CSF 10 mcg/kg subcutaneously, daily, which started at least 24 hours after the last dose of chemotherapy and continued until the completion of apheresis.
Per the institutional protocol, all patients return to the clinic the day after chemotherapy to receive information regarding chemotherapy schedule, chemotherapy side effects, G-CSF schedule, G-CSF side effects, and G-CSF administration technique, and to receive their first dose of home supply of G-CSF. Day 1 was defined as the date the patient received the first dose of chemotherapy. At day 9, a white blood cell (WBC) count was obtained, and if the WBC count was >2 × 109/L, then a CD34+ cell count was obtained. If the CD34+ cell count was ≥20/mcL, then the patient proceeded with apheresis.
If on day 9 the WBC count was <2 × 109/L, then G-CSF treatment was continued, and, per institutional protocol, the WBC count was evaluated again on day 12. If the WBC count was >2 × 109/L on day 12, then a CD34+ cell count was obtained; if this CD34+ cell count was ≤20/mcL, then plerixafor was initiated at 0.24 mg/kg (or was adjusted per the prescribing information for renal sufficiency), daily, until the completion of collection (ie, plerixafor maximum dose, 24 mg). The patient proceeded to apheresis once the CD34+ cell count was ≥20/mcL.
The institutional collection protocol required a minimum collection of 2 × 106 CD34+ cells/kg, which is an accepted minimum cell dose to proceed to autologous HCT.3 However, our institution-specific cell dose target for patients with lymphoma was 4 × 106 CD34+ cells/kg.
Efficacy and Safety Outcomes
The primary efficacy outcome was the rate of successful stem-cell collection, which was defined as a yield of ≥2 × 106 CD34+ cells/kg in ≤2 apheresis sessions. The secondary efficacy end points included the rate of optimal stem-cell collection, defined as a yield of ≥4 × 106 CD34+ cells/kg in a maximum of 2 apheresis sessions, the median number of CD34+ cells/kg collected per individual apheresis session, the absolute number of CD34+ cells/kg collected per patient over all apheresis sessions, the incidence of plerixafor use, the time between chemomobilization and the first apheresis session, and the time between chemomobilization and the last apheresis session.
The secondary safety end points included the incidence of hemorrhagic cystitis, the incidence of febrile neutropenia, and the incidence of emergency department visits and hospitalizations between the start of chemomobilization and the completion of apheresis.
The duration of posttransplant hospitalization and the time to neutrophil and platelet engraftment after autologous HCT were hypothesis-generating end points to ensure that changing chemomobilization agents did not have any unanticipated impact on the transplant course. Although no differences were expected, even slight differences in the duration of hospitalization or engraftment timing between the 2 groups would have spurred the need for additional evaluation to determine whether adverse events related to a specific chemomobilization agent were a contributing factor, which may have led our institution to favor one agent over another.
The time to neutrophil engraftment was defined as the first of 3 consecutive values on different days that the absolute neutrophil count was ≥0.5/mcL.11 The time to platelet engraftment was defined as the first of 3 consecutive values on different days that the platelet count was >50 × 109 L in the absence of platelet transfusion in the previous 7 days.12
Statistical Methods
Descriptive statistics were used to summarize the baseline patient characteristics and clinical outcomes. A two 1-sided test was used to assess whether cyclophosphamide is inferior to etoposide in the rate of successful stem-cell collection. Agresti-Caffo 90% confidence intervals (CIs) are reported for the difference in proportions. Cyclophosphamide was considered noninferior to etoposide if the resulting CIs were higher than the noninferiority threshold of –10%. Superiority tests were used to compare other variables between the drug groups. Continuous variables were analyzed using a 2-sided t-test or a Mann-Whitney U test as appropriate, and categorical data were assessed using a Pearson’s chi-squared test or a Fisher’s exact test as appropriate.
The times to neutrophil and platelet engraftments were evaluated using a log-rank test. Assuming estimated proportions of 95% and 90% and a noninferiority margin of 10% in the primary end point, a test of noninferiority required a sample size of at least 38 patients per treatment group for 80% power (type I error rate of 5%). All statistical testing was 2-sided, with an a priori significance level of 0.05 (P <.05). All statistical analyses were performed using GraphPad Prism version 8.0 (GraphPad Software, Inc; La Jolla, CA) and R for Windows version 3.6.3 using the DescTools package.
Results
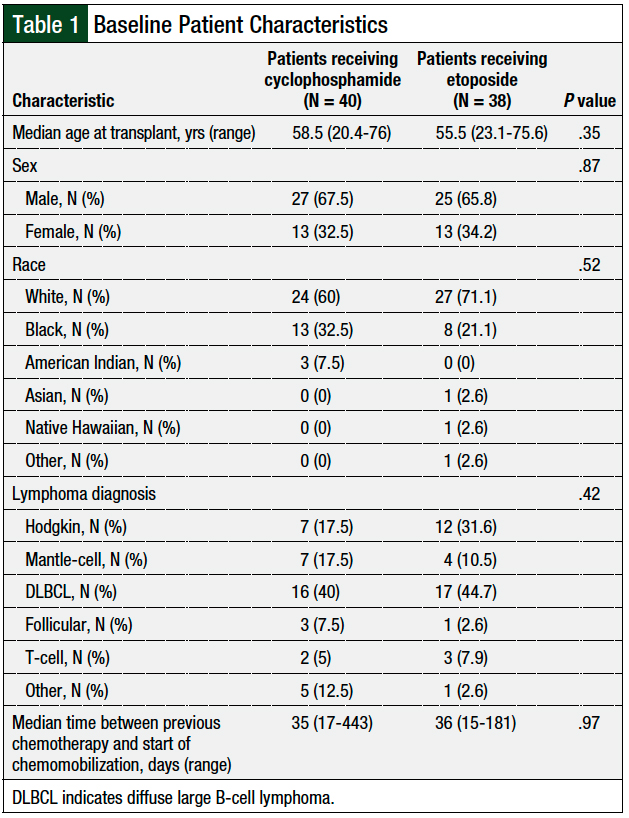
A total of 79 patients were initially identified based on the study’s inclusion criteria. One patient received cyclophosphamide at a dose of 4 g/m2 and was subsequently excluded from analysis. The baseline characteristics, including age, sex, lymphoma diagnosis, and the time from the last chemotherapy to the initiation of chemomobilization, were balanced between the cyclophosphamide and etoposide groups (Table 1).
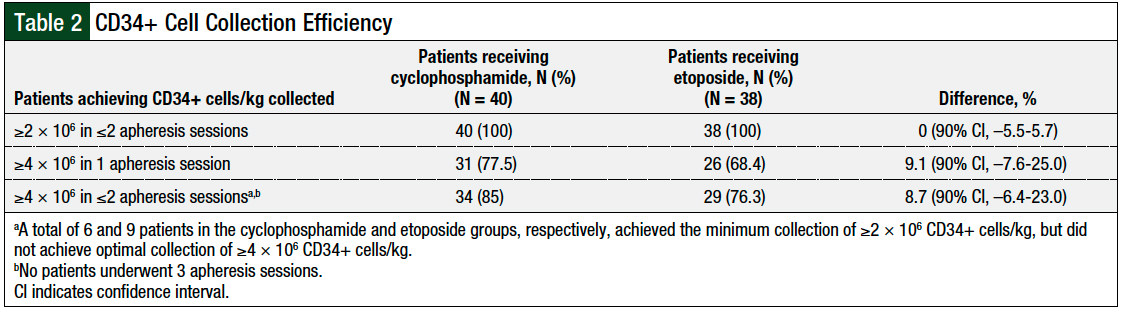
All patients in both groups met the study’s primary end point and had a collection of ≥2 × 106 CD34+ cells/kg in ≤2 apheresis sessions (difference, 0%; 90% CI, –5.5%-5.7%; Table 2). Cyclophosphamide was noninferior to etoposide in the rates of successful collection of ≥4 × 106 CD34+ cells/kg in 1 apheresis session (difference, 9.1%; 90% CI, –7.6%-25%), as well as the successful collection of ≥4 × 106 CD34+ cells/kg in 2 apheresis sessions (difference, 8.7%; 90% CI, –6.4%-23%; Table 2).
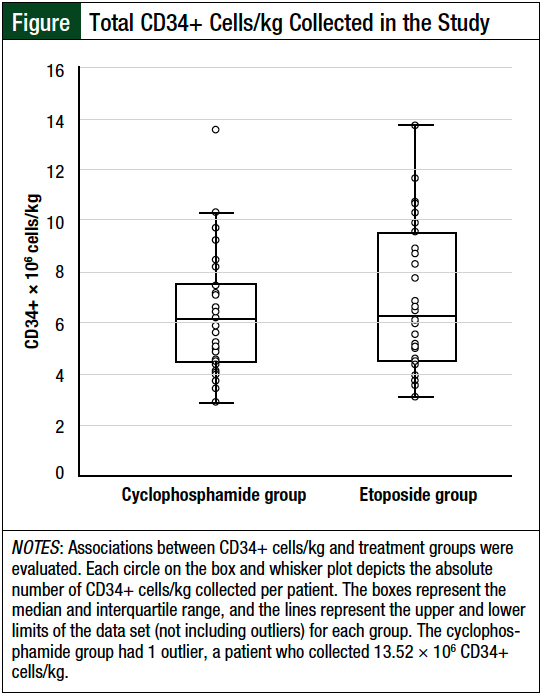
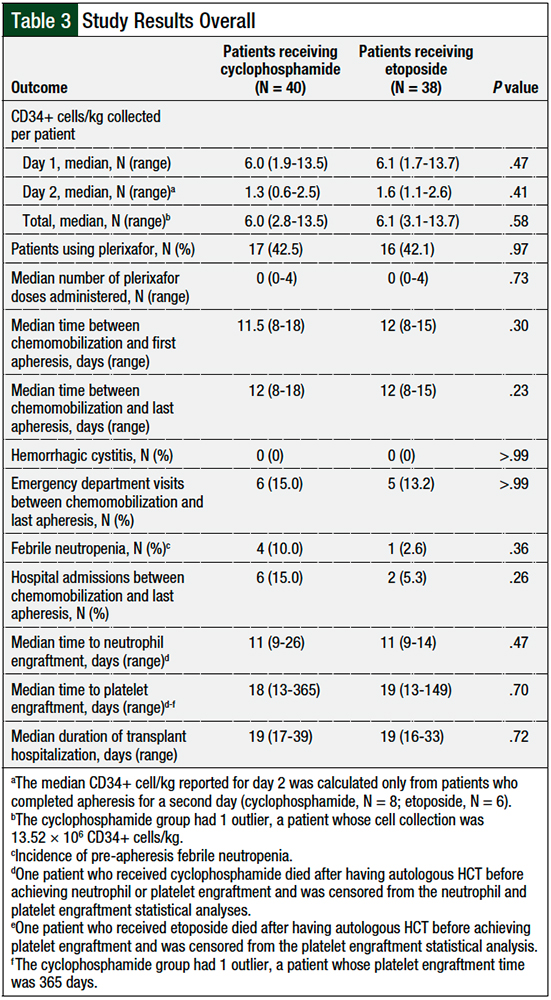
Overall, 6 patients in the cyclophosphamide group and 9 patients in the etoposide group met the primary end point of a collection of ≥2 × 106 CD34+ cells/kg, but they did not achieve ≥4 × 106 CD34+ cells/kg in 2 apheresis sessions. The median number of CD34+ cells/kg per patient collected was similar between the 2 groups—cyclophosphamide, 6.0 × 106 cells/kg versus etoposide, 6.1 × 106 cells/kg (P = .58; Figure and Table 3).
Plerixafor use was similar in the 2 groups (cyclophosphamide, 42.5% vs etoposide, 42.1%; P = .97; Table 3). The median times between chemomobilization and first apheresis session (cyclophosphamide, 11.5 days vs etoposide, 12 days; P = .30; Table 3) and last apheresis session (cyclophosphamide, 12 days vs etoposide, 12 days; P = .23; Table 3) were also similar.
No patients in either group had hemorrhagic cystitis. There were similar rates of emergency department visits related to cyclophosphamide (N = 6; 15%) and to etoposide (N = 5; 13.2%; P >.99; Table 3) use in the 2 groups. More patients who received cyclophosphamide mobilization had febrile neutropenia (N = 4; 10%) between chemotherapy and apheresis compared with etoposide (N = 1; 2.6%; P = .36; Table 3), as well as more hospital admissions with cyclophosphamide (N = 6; 15%) versus etoposide (N = 2; 5.3%; P = .26; Table 3); however, these differences were not significant.
Hospitalizations in the cyclophosphamide group resulted from fever (N = 4) and syncope (N = 2); in the etoposide group hospitalizations were caused by hypoxia (N = 1) and gastrointestinal bleeding (N = 1).
The median time to posttransplant neutrophil engraftment (cyclophosphamide, 11 days vs etoposide, 11 days; P = .47; Table 3) and platelet engraftment (cyclophosphamide, 18 days vs etoposide, 19 days; P = .70; Table 3) after autologous HCT was similar between the 2 groups. The median duration of transplant-related hospitalization was also similar in the 2 groups (cyclophosphamide, 19 days vs etoposide, 19 days; P = .72; Table 3).
Discussion
Mobilization and collection of an adequate cell dose before autologous HCT are essential for patients with lymphoma, given that poor mobilization is predictive of reduced survival.13,14 The optimal drug and dose for successful mobilization have not been clearly established. One prospective observational study of 79 patients with non-Hodgkin lymphoma (NHL) examined the use of cyclophosphamide 4 g/m2 and showed that more than 80% of patients had a successful collection of ≥1.5 × 106 CD34+ cells/kg in a single apheresis session.15 However, 21.5% of patients in that study were aged ≥60 years, and the rate of neutropenic fever was 53% in those patients and 45% in patients aged <60 years.15
A retrospective study evaluating patients with NHL who received cyclophosphamide 4 g/m2 or 2 g/m2 showed improved mobilization efficacy with the higher dose of cyclophosphamide at the expense of significantly increased rates of adverse events.16 Patients in the cyclophosphamide 4-g/m2 group had a collection of ≥2 × 106 CD34+ cells significantly more often than patients in the cyclophosphamide 2-g/m2 group (96% vs 68%, respectively; P = .0116). Patients in the cyclophosphamide 4-g/m2 group also had a significantly higher rate of hospitalizations for febrile neutropenia than patients in the cyclophosphamide 2-g/m2 group (32% vs 0%, respectively; P = .018). When evaluating the cyclophosphamide 2-g/m2 group, this study had worse mobilization efficacy, despite using the same cyclophosphamide dose and definition of successful collection as in our study.16 These differences in efficacy may be a result of the delayed G-CSF start date for these patients (G-CSF was not started until day 4 after chemomobilization16).
Etoposide has also proved effective for chemomobilization.7 A retrospective study examining patients between 2004 and 2010 evaluated etoposide 375 mg/m2 daily for 2 days and had results comparable to our study results.7 The researchers observed that 159 patients collected a median of 6.2 × 106 CD34+ cells/kg, with 94% of patients collecting >2 × 106 CD34+ cells/kg, and only 6% of patients required admission during the mobilization period.7 The dose of etoposide used for chemomobilization also affects the rates of efficacy.
A nonrandomized, prospective cohort study with 63 patients undergoing chemomobilization, 31 of whom had lymphoma, examined high-dose etoposide ranging from 1600 to 2000 mg/m2.17 The median CD34+ cell collection ranged from 4.7 × 106 to 6.5 × 106 CD34+ cells/kg. However, 37% of the patients were hospitalized after chemomobilization, with sepsis being the primary reason for hospitalization. The hospitalization rate was significantly lower in patients receiving 1600 mg/m2 of etoposide than in those receiving 2000 mg/m2 of etoposide (19% vs 50%, respectively; P = .03), but the incidence was still higher than the rates in our study.17
A few studies compared the efficacy and safety outcomes of cyclophosphamide with etoposide, but none has used the exact same dosing strategy as our study. However, Milone and colleagues compared etoposide 200 mg/m2 daily for 3 days combined with G-CSF 16 mcg/kg daily, with cyclophosphamide 4 g/m2 combined with G-CSF 10 mcg/kg daily.18
The efficacy outcome of mean CD34+ cells collected favored the etoposide group compared with the cyclophosphamide group (11 × 106 cells/kg vs 6.3 × 106 cells/kg, respectively; P = .004), but the safety analysis was similar between the 2 groups. Febrile neutropenia occurred in 7.3% of patients in the etoposide group, whereas 5.4% of patients in the cyclophosphamide group had serious adverse events, including 1 case of hemorrhagic cystitis and 1 case of dilated myocardiomyopathy.18
Although the mean number of hospitalization days when administering the mobilization regimens was higher in the cyclophosphamide group than in the etoposide group (3.9 days vs 0.2 days, respectively; P = .0001), this was driven by required hospital admission for IV hydration for patients in the cyclophosphamide group to prevent hemorrhagic cystitis.18
Because our institution administers cyclophosphamide and IV hydration to patients in the outpatient setting, our results did not demonstrate the same increase in cyclophosphamide-related hospitalization rates. Furthermore, administering a single dose of cyclophosphamide instead of multiple daily etoposide infusions decreases the total amount of time a patient spends in a clinic.
Another smaller retrospective study of 60 patients showed similar efficacy results for cyclophosphamide and etoposide chemomobilizations.19 The study compared cyclophosphamide 4 g/m2 with etoposide 500 mg/m2 daily for 2 days. Of the 31 patients in the cyclophosphamide group, 6 (19%) patients did not have a collection of at least 2 × 106 CD34+ cells/kg; by contrast, there were no patients in the etoposide group who did not have a collection of at least 2 × 106 CD34+ cells/kg (P = .051).19
Although patients in the cyclophosphamide group collected significantly fewer total CD34+ cells than those in the etoposide group (4.44 × 106 cells/kg vs 16.22 × 106 cells/kg, respectively; P <.001), the etoposide group also had numerically higher rates of febrile neutropenia (67% vs 58%, respectively; P = .454). The time to neutrophil engraftment between the cyclophosphamide and etoposide groups was the same, at 10 days, and no significant difference was seen between the time to platelet engraftment between the 2 groups (11 days vs 10 days, respectively; P = .272).19
The lower dose of cyclophosphamide 2 g/m2 used in our study is effective and safe compared with a dose of cyclophosphamide 4 g/m2 used in previous studies.15,19 Those studies reported higher rates of neutropenic fever than was observed in our study, although direct comparisons are not possible because of differences in trial design and study population.15,19 The higher dose of etoposide used in these studies might have contributed to the difference in CD34+ cells collected compared with our study.15,19
In our retrospective study, a single dose of only cyclophosphamide 2 g/m2 was used to quantify efficacy and safety compared with our institution’s previous standard of etoposide-based chemomobilization and was noninferior to etoposide. The patients in the etoposide and cyclophosphamide groups were evenly matched, and patients with a variety of lymphoma diagnoses were included.
Limitations
Our study had several limitations. Because of the retrospective nature of this study, we extracted data via a manual chart review, which is limited by the extent to which information is documented in the electronic medical record.
Data surrounding the exact timeline of G-CSF initiation after the last dose of chemotherapy were not collected, although all patients at our institution always receive their first dose of G-CSF at a clinic appointment, where injection technique can be observed by a registered nurse.
Furthermore, our algorithm for plerixafor use, WBC count, and CD34+ cell count monitoring is institution-specific, thus variability between the institutional protocols may affect the results.
Our data could also be limited by unidentified confounders, which is inherent to this study’s retrospective design. No efficacy or safety outcomes were significantly different between the cyclophosphamide and etoposide groups in our study, and all patients had successful chemomobilization collections based on accepted definitions established in the literature.3-5
The rates of febrile neutropenia and hospitalizations in our study were similar after either cyclophosphamide or etoposide mobilization and were less than or consistent with the rates of febrile neutropenia published in other studies.15,16,18,19
Conclusion
Our results indicate that chemomobilization with a single dose of cyclophosphamide 2 g/m2 is noninferior to chemomobilization with etoposide 300 mg/m2 daily for 2 days. No differences were observed in the rates of successful stem-cell collection or adverse events between patients with lymphoma chemomobilized with these specific cyclophosphamide and etoposide regimens. These results demonstrate the safety and efficacy of chemomobilization with a single dose of cyclophosphamide 2 g/m2, a dose that is not often supported in the previously published literature. Therefore, we have continued chemomobilization with single-dose cyclophosphamide at our institution. Nonetheless, both regimens are reasonable options for chemomobilization in patients with lymphoma.
Our results should be confirmed in a larger randomized, controlled study that directly compares the efficacy and safety outcomes of a single dose of cyclophosphamide 2 g/m2 with etoposide 300 mg/m2 daily for 2 days when used for chemomobilization in patients with lymphoma.
Acknowledgments
We would like to thank Daniel J. Crona, PharmD, PhD, CPP, and Allison M. Deal, MS, for their assistance with statistical test choice and confirmation of results.
Author Disclosure Statement
Dr Dennison, Dr Pedretti, Dr Zhu, Dr Stocker, Dr Shaw, Dr Grgic, Ms Heiling, and Dr Ptachcinski have no conflicts of interest to report. Dr Alexander is on the Advisory Board of Teva Pharmaceuticals, AcelRx, Sanofi Genzyme, Jazz Pharmaceuticals, and Juno Therapeutics. Dr Riches is a Medical Monitor for Gamida Cell.
References
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813-1826.
- Giralt S, Costa L, Schriber J, et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant. 2014;20:295-308.
- Duong HK, Savani BN, Copelan E, et al. Peripheral blood progenitor cell mobilization for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2014;20:1262-1273.
- Güner ŞI, Yanmaz MT, Selvi A, Usul C. The high effect of chemomobilization with high-dose etoposide + granulocyte-colony stimulating factor in autologous hematopoietic peripheral blood stem cell transplantation: a single center experience. Hematol Rep. 2016;8:18-22.
- Mohammadi S, Mohammadi AM, Nikbakht M, et al. Optimizing stem cells mobilization strategies to ameliorate patient outcomes: a review of guidelines and recommendations. Int J Hematol Oncol Stem Cell Res. 2017;11:78-88.
- Chow E, Rao KV, Wood WA, et al. Effectiveness of an algorithm-based approach to the utilization of plerixafor in patients undergoing chemotherapy-based stem cell mobilization. Biol Blood Marrow Transplant. 2014;20:1064-1068.
- Wood WA, Whitley J, Goyal R, et al. Effectiveness of etoposide chemomobilization in lymphoma patients undergoing auto-SCT. Bone Marrow Transplant. 2013;48:771-776.
- Watts MJ, Ings SJ, Leverett D, et al. ESHAP and G-CSF is a superior blood stem cell mobilizing regimen compared to cyclophosphamide 1.5 g m–2 and G-CSF for pre-treated lymphoma patients: a matched pairs analysis of 78 patients. Br J Cancer. 2000;82:278-282.
- Mollee P, Pereira D, Nagy T, et al. Cyclophosphamide, etoposide and G-CSF to mobilize peripheral blood stem cells for autologous stem cell transplantation in patients with lymphoma. Bone Marrow Transplant. 2002;30:273-278.
- Mahindra A, Bolwell BJ, Rybicki L, et al. Etoposide plus G-CSF priming compared with G-CSF alone in patients with lymphoma improves mobilization without an increased risk of secondary myelodysplasia and leukemia. Bone Marrow Transplant. 2012;47:231-235.
- Center for International Blood and Marrow Transplant Research. Forms Instruction Manual. Updated January 25, 2021. www.cibmtr.org/manuals/fim/1/en/topic/q8-11-initial-anc-recovery. Accessed June 15, 2020.
- Ninan MJ, Flowers CR, Roback JD, et al. Posttransplant thrombopoiesis predicts survival in patients undergoing autologous hematopoietic progenitor cell transplantation. Biol Blood Marrow Transplant. 2007;13:895-904.
- Tomblyn M, Burns LJ, Blazar B, et al. Difficult stem cell mobilization despite adequate CD34+ cell dose predicts shortened progression free and overall survival after autologous HSCT for lymphoma. Bone Marrow Transplant. 2007;40:111-118.
- Pavone V, Gaudio F, Console G, et al. Poor mobilization is an independent prognostic factor in patients with malignant lymphomas treated by peripheral blood stem cell transplantation. Bone Marrow Transplant. 2006;37:719-724.
- Jantunen E, Mahlamäki E, Nousiainen T. Feasibility and toxicity of high-dose chemotherapy supported by peripheral blood stem cell transplantation in elderly patients (≥60 years) with non-Hodgkin’s lymphoma: comparison with patients <60 years treated within the same protocol. Bone Marrow Transplant. 2000;26:737-741.
- Sizemore CA, Laporte J, Holland HK, et al. A comparison of toxicity and mobilization efficacy following two different doses of cyclophosphamide for mobilization of hematopoietic stem cells in Non-Hodgkin’s Lymphoma patients. Biol Blood Marrow Transplant. 2010;16(suppl 2):S206. Abstract 130.
- Kanfer EJ, McGuigan D, Samson D, et al. High-dose etoposide with granulocyte colony-stimulating factor for mobilization of peripheral blood progenitor cells: efficacy and toxicity at three dose levels. Br J Cancer. 1998;78:928-932.
- Milone G, Leotta S, Battiato K, et al. Intermediate dose etoposide plus G-CSF 16 µg/kg is more effective than cyclophosphamide 4 g/m2 plus G-CSF 10 µg/kg in PBSC mobilization of lymphoma patients. Leuk Lymphoma. 2007;48:1950-1960.
- Hyun SY, Cheong JW, Kim SJ, et al. High-dose etoposide plus granulocyte colony-stimulating factor as an effective chemomobilization regimen for autologous stem cell transplantation in patients with non-Hodgkin lymphoma previously treated with CHOP-based chemotherapy: a study from the Consortium for Improving Survival of Lymphoma. Biol Blood Marrow Transplant. 2014;20:73-79.